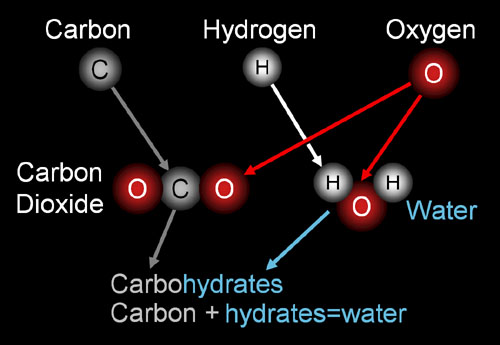
Primarily, Carbohydrates are called such because it contains essential hydrates of carbon and are composed of water and carbon. Carbohydrates can be given by the equation (CH2O) n.
naming the biomolecule
Carbohydrates have four distinct functions. These are,
1. Used as a storage medium (starch in plants and glycogen in animals)
2. Used as an energy source since it can be broken down easily
3. Used as a Precursor Molecule in the synthesis of certain bio molecules
4. Has a structural function (Cellulose in plants and Chitin in exoskeletons or insects)
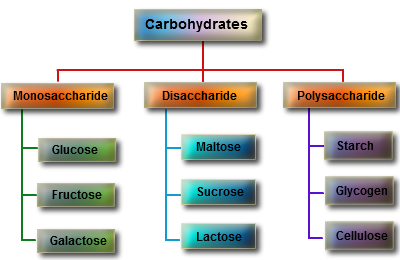
These biomolecules are classed as Monosaccharide, Disaccharide, Oligosaccharides and Polysaccharides. Each is structurally different in terms of the amount of simple sugars it contains.
Monosaccharide simple sugars with multiple –OH groups bonded on a number of carbons.
Disaccharide -2 monosaccharide covalently linked.
Oligosaccharides- few monosaccharides linked covalently together.
Polysaccharides – polymers consisting of chains of monosaccharide units.
The bond which is formed between Carbohydrates is called a Glycosidic bond . These biomolecules can be classed into Reducing and Non-reducing sugars. A Reducing sugar is a carbohydrate that serves as a reducing agent due to its free aldehyde or ketone functional groups in its molecular structure. Glucose, fructose, lactose, and maltose are examples of reducing sugars since these have free aldehyde or ketone functional groups in its molecular structure. A Non-reducing sugar is a carbohydrate that is not easily oxidized by a weak oxidizing agents in basic aqueous solution. In basic aqueous solution, non-reducing sugars do not generate compounds containing an aldehyde group. One such example is sucrose, which contains neither a hemiacetal group nor a hemiketal group.
In terms of the video, “TheNewBoston” introduces the topic as carbohydrates and makes an important note that we have learnt before- Carbohydrates are biomolecules. He also states that the Human Body “loves” Carbon, which makes up the back bone of a carbohydrate. He implies that the body actively uses molecules made of carbon. Describing carbohydrate structure, he illustrates using a drawing the ratio to which carbohydrates is made up, which is a 1:2:1 ratio, in terms of Carbon, Hydrogen and Oxygen respectively. In the video, “TheNewBoston” only makes a slight suggestion to where we, humans, obtain carbohydrates. He also describes that we use carbohydrates as a source of energy, also giving the example Glucose and how it used in a brief description.
How are carbohydrates classified?
These biomolecules are classed as Monosaccharide, Disaccharide, Oligosaccharides and Polysaccharides. Each is structurally different in terms of the amount of simple sugars it contains.
Monosaccharide -simple sugars with multiple –OH groups bonded on a number of carbons.
Disaccharide -2 monosaccharide covalently linked.
Oligosaccharide- few monosaccharides linked covalently together.
Polysaccharides – polymers consisting of chains of monosaccharide units.
Carbohydrate Class
Credits to Biochem
students Carlos, Shenelle, Liniker, Latisha, et. al
The bond which is formed between Carbohydrates is called a Glycosidic bond . Note its position on the diagram. In Carbohydrates part 1 we mentioned the importance of Carbohydrates and structure. When carbohydrates bond, its functions change.
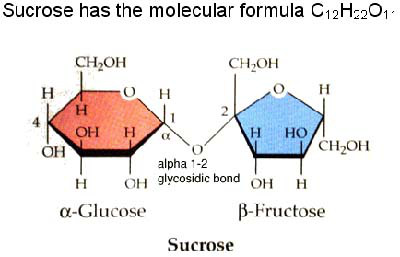
Diagram with Glycosidic Bond
References:
“Nonreducing Sugar.” OChemPal. science.uvu.edu/ochem/index.php/alphabetical/m-n/nonreducing-sugar/.
“Tollen’s Test.” OChemPal. science.uvu.edu/ochem/index.php/alphabetical/s-t/tollens-test/.
“What is the principle of the Fehling’s test.” The Q&A wiki. http://wiki.answers.com/Q/What_is_the_principle_of_the_Fehling’s_test.
http://images.tutorvista.com/cms/images/38/structure-of-carbohydrates.png
http://12angrymen.files.wordpress.com/2008/06/sucrose.jpg?w=510
What are tests for Reducing sugar and Non-reducing sugars?
A Reducing sugar is a carbohydrate that serves as a reducing agent due to its free aldehyde or ketone functional groups in its molecular structure. Glucose, fructose, lactose, and maltose are examples of reducing sugars since these have free aldehyde or ketone functional groups in its molecular structure.
A Non-reducing sugar is a carbohydrate that is not easily oxidized by a weak oxidizing agents in basic aqueous solution. In basic aqueous solution, non-reducing sugars do not generate compounds containing an aldehyde group. One such example is sucrose, which contains neither a hemiacetal group nor a hemiketal group.
Primarily, one can use a series of tests to differentiate carbohydrates. Benedict’s reagent is used to determine the presence of a reducing sugar, i.e. when a mixture contains sugar it will turn from blue to green to orange to red after this test – an indication that the reducing sugar is present. Fehling’s reagent also has the same result. If the mixture stays blue, it is a Disaccharide. One can further perform the Modified Barfoed’s test to indicate if the reducing sugar from the Benedict’s test is either Disaccharide or a Monosaccharide. This can be followed by the Seliwanoff’s test to determine if the sugar is a ketose or an aldose sugar. If the sugar is a ketose sugar, the mixture will turn pink while if the sugar is an aldose sugar, the mixture is colourless. In addition one can determine if the sugar is pentose or not by performing the Bail’s test. A positive test results in the mixture turning green while a negative result results in the mixture remaining yellow.

0 comments:
Post a Comment