
MORE ON PROTEINS AND AMINO ACIDS!
Amino Acids and Proteins were one of the 3 bio-molecules we studied in class. Amino acids are defined as a simple organic compound containing both a carboxyl (COOH) and an amino (NH2) group. Proteins are defined as any of a class of nitrogenous organic compounds that consist of large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms. Amino acids have the basic structure seen in the diagram where R can be given by different groups (highlighted in red). The central carbon is referred to as the Alpha carbon.
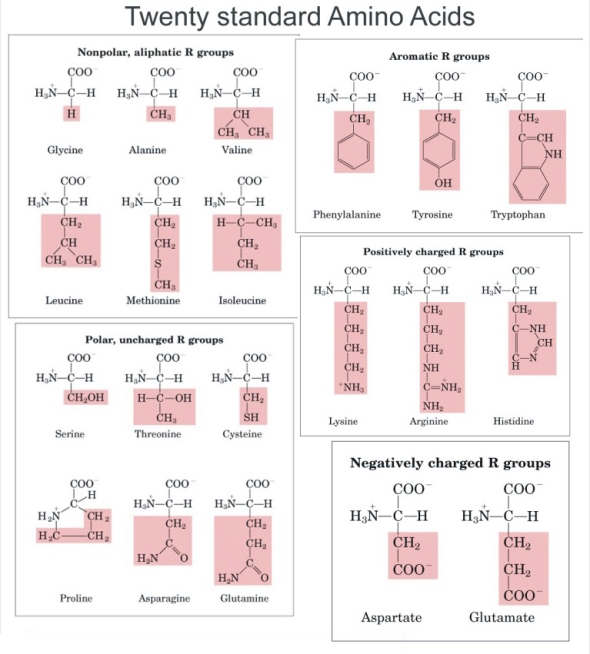
There are 20 essential Amino acids for survival which can be classed as either Essential or Non-Essential Amino Acids.
An Essential amino acid is one that is required by animals that cannot be synthesize by them and must be supplied in the diet. (marked)
Non essential amino acids are those that are synthesized by the body. (unmarked)
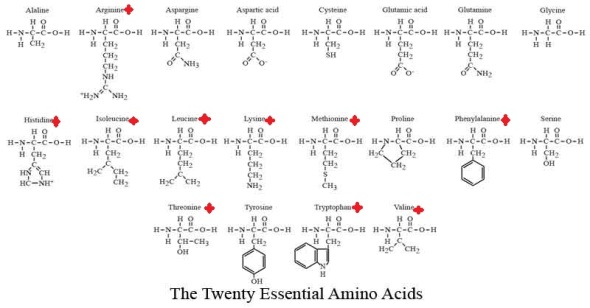
Furthermore Essential Amino Acids can be described as either Complete Proteins or Incomplete Proteins. Complete proteins contain all 10 essential amino acids and are derived from animal sources while Incomplete proteins lack one or more of the essential amino acids and most a vegetable based.
How are Amino acids and Proteins classed by structure?
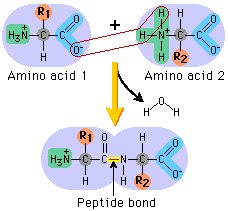
Amino acids bond together to from Proteins. These bonds are called peptide bonds. As a result of bonding Amino acids can be arranged into the following long chain structures;
Peptide- short polymer of amino acids
Di-peptide- contains 2 amino acids joined by a peptide bond.
Tri-peptide- a molecule with 3 amino acids joined by peptide bonds.
Polypeptide- a macro-molecule containing many amino acids.
Protein- a biological molecule of molecular weight 5000g/mol that is made up of polypeptide chains.
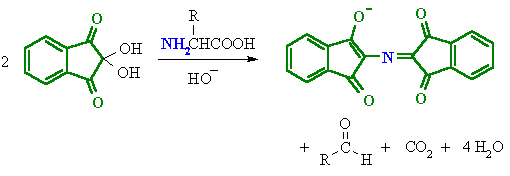
How can we identify Amino Acids from Proteins?
We can use to chemical tests to determine which compounds are amino acids or proteins. These are the Ninhydrin Reaction and the Biuret Test.
Ninhydrin reacts with amino acids to from a Purple colour imino derivative. This derivative is a positive test for amino acids which are commonly colourless.
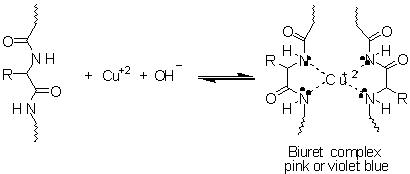
The Biuret test involves using biuret reagent which is light blue in colour. It contain Copper(II) ion in an alkaline solution. Biuret turns purple when mixed with a solution contain proteins. Biuret reagent interacts with peptide bonds of proteins. The purple colour formed is a positive test for proteins.
Credits to Biochem
students Carlos, Shenelle, Liniker, Latisha, et. al
How are Proteins classed in terms of structure?
Proteins are classed by the arrangement and the number of polypeptide chains it contains and the level of folding that occur. Protein folding occurs due to Hydrophobic Interactions, Ionic Bonding, Hydrogen bonding and Disulphide bonding. Protein structure can be either Primary, Secondary, Tertiary or Quaternary.
Primary structure: the linear arrangment of amino acids in a protein and the location of covalent linkages such as disulfide bonds between amino acids. These Disulphide bonds are not denatured.
Secondary structure: areas of folding or coiling within a protein; examples include alpha helices and Beta pleated sheets, which are stabilized by hydrogen bonding.
Tertiary structure: the final three-dimensional structure of a protein, which results from a large number of non-covalent interactions between amino acids.
Quaternary structure: non-covalent interactions that bind multiple polypeptides into a single, larger protein. Hemoglobin has quaternary structure due to association of two alpha globin and two beta globin polyproteins.

Protein Structure
Folding can be denatured in several ways.
Heat and Ultra Violet Radiation-Hydrogen bonds are broken by increased translational and vibration energy
Strong Acids/Bases- salt formation, disruption of hydrogen bonds
Urea- competion for hydrogen bonds
Agitation-shared hydrogen bonds
Some Organic Solvents- change in dielectric constant and hydration of ionic groups.
Proteins are essential to body function and are useful in life processes. An example of an important protein structure is an Enzyme.
“Essential Amino acid.” wordnet web. wordnetweb.princeton.edu/perl/webwn?s=essential%20amino%20acid.
“The Structure of Proteins.” arbl.cvmbs.colostate.edu. http://www.vivo.colostate.edu/hbooks/genetics/biotech/basics/prostruct.html.


0 comments:
Post a Comment